Hydrophilic Interaction Chromatography (HILIC)
This is a variant of normal phase chromatography that is performed with a very polar stationary phase and a mostly organic mobile phase. When the mobile phase contains > 60% organic solvent, then hydrophilic interaction becomes significant. With neutral materials such as PolyHYDROXYETHYL A™, this is the only significant force involved. With ion-exchange columns, hydrophilic interaction will be superimposed on the electrostatic effects. See the example of this with Histone H1 phosphorylation variants.
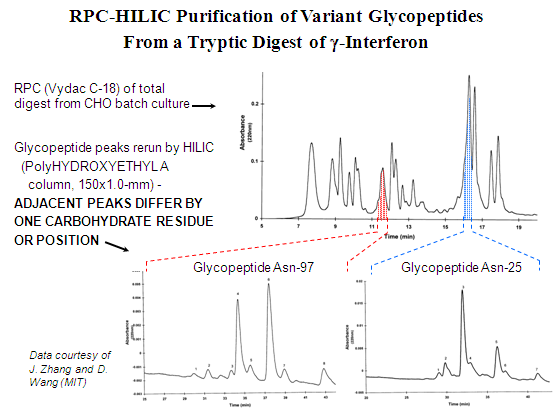
Selectivity in HILIC is the opposite of that of RPC. It is very sensitive to addition or deletion of a Ser- or carbohydrate residue, less sensitive to addition or deletion of a Leu- or Phe- residue. Thus, the two modes are complementary and are sometimes used in sequence to purify complex mixtures. An example is the assessment of the degree of glycation of γ-interferon. The tryptic fragments are resolved on a RPC column. The two glycopeptide peaks are collected and rerun via HILIC on a PolyHYDROXYETHYL A™ column. This resolves each peak into a library of glycopeptide peaks, each differing from its neighbor by one carbohydrate residue.
Basic residues are the most hydrophilic, followed by phosphorylated residues. Next comes Asn-, Ser-, etc. with Phe- and Leu- being the least hydrophilic.
The more polar a stationary phase, the less organic solvent necessary to get a given degree of retention. PolyHYDROXYETHYL A™ was designed to afford superior results in HILIC. Select the pore diameter that matches your application:
1) 60- or 100-Å: Small solutes in general. See Metabolomics. The 3-µm, 100-Å material is particularly useful.
2) 200- or 300-Å: Peptides and most proteins; di- and trinucleotides (ADP; NADH; etc.)
3) 1000-Å: Extremely polar solutes such as ATP and aminoglycoside antibiotics (see Metabolomics).
Salts: Essential to get reproducible retention times and symmetrical peaks. Usually, 10-15 mM suffices unless the solute is highly charged. Ammonium acetate or formate can be used if salt must be volatile. If the absorbance is to be monitored at low wavelengths such as 215 nm, then use triethylamine phosphate (TEAP) or sodium methylphosphonate. In cases where the solute is extremely well retained, such as intact proteins, try 50 mM formic acid as the additive.
pH: Retention of peptides is maximal around pH 3. This is because they have a net + charge at that pH, and basic solutes are the most polar. Solutes that are not electrolytes are less sensitive to pH. NOTE: Selective isolation of glycopeptides: Dr. Steven Carr (Broad Institute) has observed that glycopeptides can be isolated from a tryptic digest with reasonable selectivity by HILIC on a PolyHYDROXYETHYL A™ column (e.g., item# 202HY0503) with a decreasing ACN gradient and 15 mM ammonium acetate right out of the bottle (pH ~ 6.5). This seems to reflect the fact that retention due to the peptide moiety decreases from pH 3 to pH 6.5, while retention due to the glycan moiety is relatively unaffected. Thus, the contribution of the glycan to retention is a greater percentage of the total, and glycopeptides elute as a class immediately after the nonglycopeptides.
Solvents: ACN and PrOH afford comparable retention. Backpressure is lower with ACN, while PrOH is a better solvent for intact proteins. An ACN:PrOH blend seems to be an even better solvent for proteins. BuOH affords even better retention than do these solvents, but its high viscosity limits the amount that can be employed.
Intact proteins: While water-soluble proteins such as cytoplasmic enzymes do not lend themselves well to HILIC, HILIC works well for proteins that do not normally occur free in aqueous solution, such as membrane proteins and histones. See Proteomics.
PolyHYDROXYETHYL A™ is a trademark of PolyLC Inc. All Rights Reserved
