Isolation of Proteins and Peptides from Extracts
and Natural Products
Generally, any single protein or peptide can be isolated in >90% purity from any crude extract by the use of two complementary modes of HPLC in sequence. If purity > 98% is desired, it may be necessary to use three modes in sequence.
PEPTIDES:
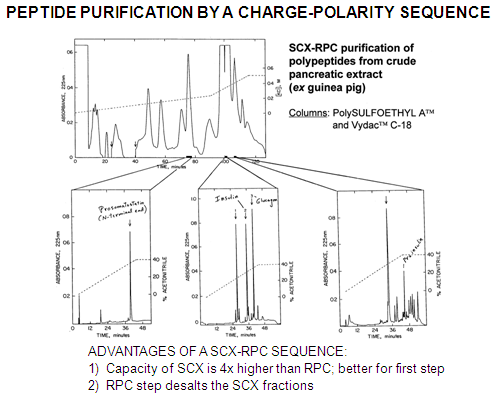
SCX-RPC: Almost all peptides will be retained by our PolySULFOETHYL A™ material at pH 2.7-3.0 and can be separated by charge differences. The fraction of interest is collected and further resolved by RPC. Peptides which coeluted with the peptide of interest in the SCX run and which presumably have similar charge-to-mass characteristics will be unlikely to have similar polarity as well and will be separated in the second dimension.
Order PolySULFOETHYL A™ now.
The SCX step should be performed first for the following reasons:
1) Ion-exchange columns have 4x higher binding capacity than a RPC column of the same size.
2) The RPC step can conveniently eliminate the salt from the SCX step.
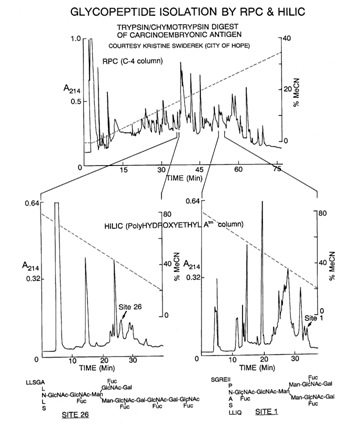
RPC-HILIC: This separates peptides first by their hydrophobic and then by their hydrophilic residue differences. Capacity is less than with SCX, but either mode can be used with mobile phases compatible with MS.
RPC-HILIC-SCX: This sequence was used to obtain polypeptides of extremely high purity from crude pituitary extracts for correction of mistaken sequence data in the literature (actually, a SCX-RPC-HILIC sequence would have been more convenient). Ref: K.F. Faull et al., Neuropeptides 32 (1998) 339.
PROTEINS:
Ion-exchange and hydrophobic interaction chromatography (HIC) are useful for most protein applications, particularly if protein tertiary structure and function is to be preserved (not a concern in proteomics). The capacity and resolving power of SEC is much less than for these modes, making it useful only in special cases. The organic solvents used for RPC and HILIC also make them useful only for special cases. Small proteins tend to work well with RPC while proteins < 30 KDa and membrane proteins frequently work well with HILIC.
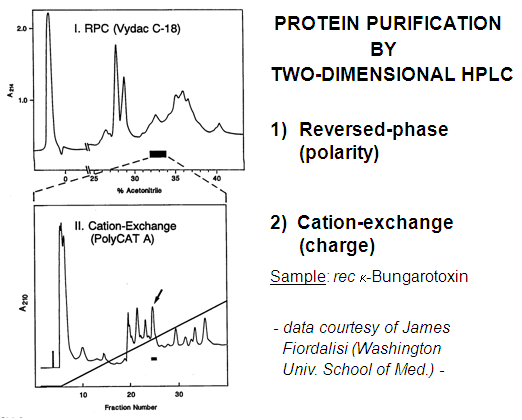
RPC-WCX: This combination worked well for this small protein. It would have been more convenient to use a WCX-RPC sequence.
HIC-IEX: This is a popular sequence because of the convenience of use of HIC to “capture” dilute proteins from a large volume. Actually, it might be more convenient to reverse the sequence. This would permit the IEX fractions to be applied directly to the HIC column upon addition of additional salt to promote binding. This separation first by polarity and then by charge is a particularly powerful combination and should be considered for fractionation of complex protein samples in proteomics analyses.
[Below] 2g of crude venom from copperhead snake (Agkistrodon contortrix contortrix)
Fibrolase Activity Fibrolase Activity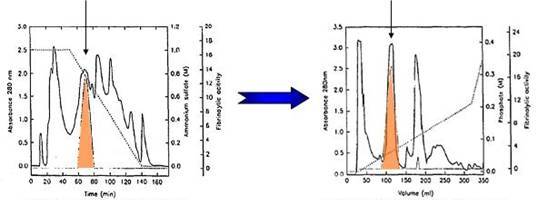
LEFT – Hydrophobic interaction chromatography (HIC) fibrolase PolyPROPYL A™ column
RIGHT – Hydroxyapatite HPLC of peak with activity collected during HIC step.
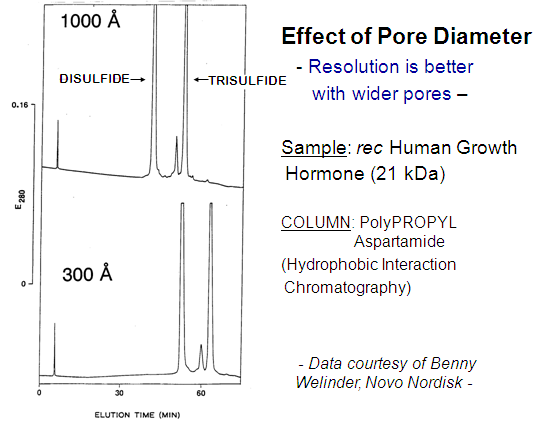
Pore Diameter: In general, stationary phases for IEX and HIC of proteins should have a pore diameter of 1000 Å rather than 300 Å. The 1000-Å materials afford sharper peaks because of the easier diffusion into and out of the pores. This is true even of a 21-KDa protein (BELOW). The lower surface area and hence capacity of 1000-Å materials is usually not a problem.
PolySULFOETHYL A™, PolyHYDROXYETHYL A™, PolyCAT A™, and PolyPROPYL A™,
are trademarks of PolyLC Inc. All Rights Reserved.
